Introduction

Food-grade PE materials are increasingly used in the food packaging industry, and brand owners now focus on both material safety and processing control during supplier selection. Xiamen Ruicheng has established a complete manufacturing chain for PE injection molded parts, covering material screening, processing stability, cleanliness management, and verification, helping customers reduce the cost of trial/errors in food-contact compliance. Food-contact safety is not determined by material alone but by whether the entire manufacturing chain can be proven safe.
The reliability of food-grade PE parts has shifted from product display to decision companionship, ensuring that every node of the procurement chain receives high-quality technical justification and compliance support.
Does PE Material for Food Packaging Meet FDA Food-Contact Requirements?
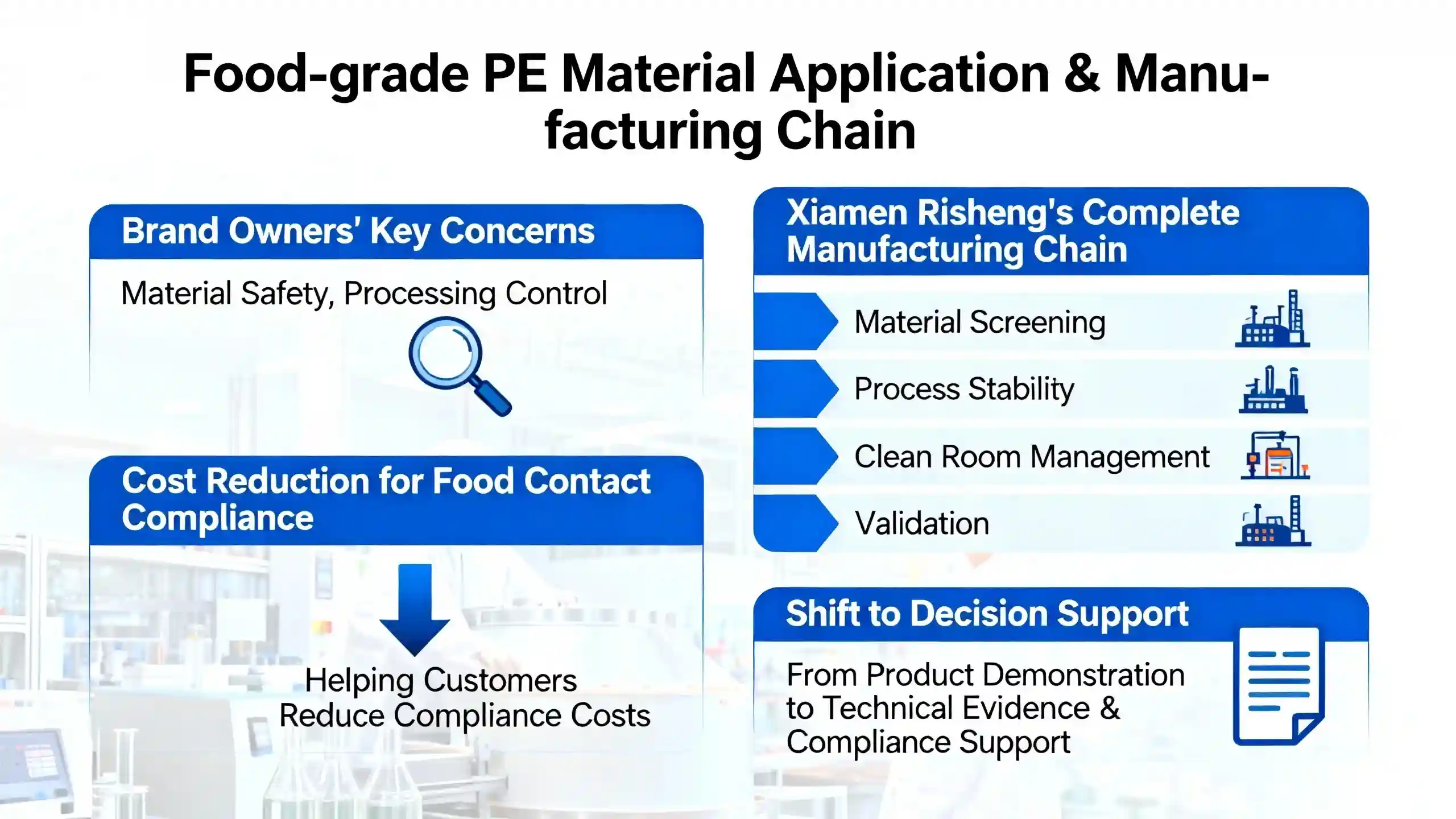
PE materials used in food packaging usually demonstrate strong compatibility with FDA food-contact requirements, especially when using FDA-compliant raw materials. Customers often focus on whether the material has migration risks, whether additives are food-grade, and whether the formula stays consistent during processing. Xiamen Ruicheng maintains batch-traceable materials and controls stable molding windows, enabling PE parts to meet food-contact expectations even under strict audits.Only when material sources are verified and traceable can food-grade PE injection parts truly satisfy FDA requirements.Different PE grades and additives may behave differently under heat or acidic environments, so proper screening and testing following FDA food-contact rules and ISO regulatory guidance are essential.
- Consistent Material Sourcing: Prevents risk caused by batch fluctuations.
- Stable Melt Flow Index: Avoids degradation and unexpected extractables.
- Transparent Additive Management: Ensures all components meet food-contact regulations.
- Traceable Documentation: Helps brands pass audits smoothly.
Do Processing Temperature and Cleanliness Determine FDA Compliance?

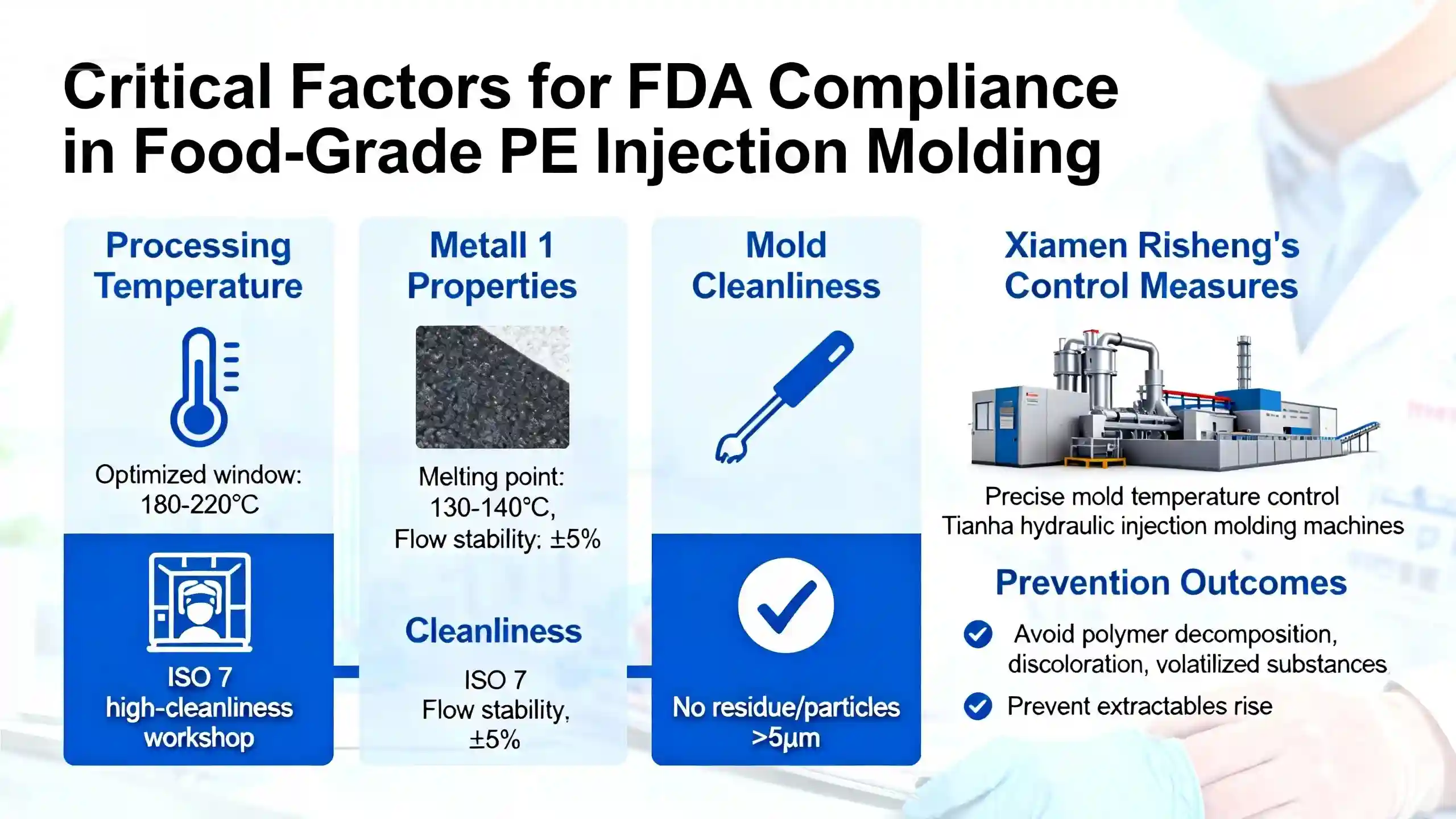
In the manufacturing of food-grade PE injection parts, melt temperature, flow stability, and mold cleanliness directly influence whether the product can pass FDA food-contact certification. Xiamen Ruicheng keeps molding temperatures within optimized processing windows to avoid decomposition, discoloration, or volatile substances. Full-chain cleanliness is one of the most decisive factors in food-grade certification.If mold cavities or the injection environment contain residues, particles, or cross-contamination, the PE part may fail FDA audits. Cleanliness must be controlled through documented checks aligned with ISO cleanliness standards and GMP production guidance.
- Stable Temperature Control: Prevents polymer breakdown and extractable rise.
- Clean Mold Surfaces: Eliminates risk of particulate contamination.
- Production Environment Monitoring: Ensures consistent hygiene levels.
- Standardized Operating Procedures: Minimizes variation caused by manual operation.
Can All Food-Application Scenarios Use PE Injection Parts That Meet FDA Standards?
Different food types—acidic foods, high-temperature foods, long-term stored foods, oily foods—pose different challenges for migration, additive stability, and mechanical reliability. Therefore, not all PE injection parts can directly claim FDA compliance without scenario-specific validation.Xiamen Ruicheng evaluates application scenarios and selects appropriate PE grades, conducting simulated migration tests, compatibility studies, and performance assessments to ensure stability in acidic media, hot-filling environments, or long-term storage. Compliance is not a certificate; it is the demonstrated safety performance in the intended application.Scenario-specific testing follows FDA’s food-contact simulation guidance and ASTM material performance standards.
- Acidic Food Compatibility: Higher migration risk requires dedicated verification.
- Heat-Exposure Stability: Additive migration increases significantly under heat.
- Long-Term Storage Conditions: Require enhanced oxidation and stability assessments.
- Structural Design Factors: Poor structure may cause cracking and contamination issues.
Comparison of FDA Requirements for Different Food Applications
| Criteria | Refrigerated Food Packaging | Ambient Food Packaging | Heated Food Packaging | Acidic Food Packaging |
|---|---|---|---|---|
| Migration Stability | High | Medium | High | High |
| Temperature Resistance | Medium | Medium | High | Medium |
| Special Formula Requirements | Medium | Low | High | High |
| Cleanroom Requirement | High | Medium | High | High |
For application-specific compliance assessment of your PE injection parts, request expert support here:contact us
How to Maintain Certified Consistency in Mass Production?
In large-scale manufacturing, maintaining FDA compliance requires ensuring that every batch of PE injection parts behaves consistently—not just sample batches. Xiamen Ruicheng employs batch-traceability, controlled processing windows, and statistical monitoring, ensuring stable performance throughout mass production.Consistency assurance determines long-term brand stability and regulatory confidence.
1.Batch Stability Control: Traceable raw-material identification ensures identical source and formula.
2.Locked Processing Windows: Prevents process drift and keeps performance stable across production lots.
3.Documentation & Traceability: Audit-ready documentation supports food-contact compliance reviews.
4.Supply-Chain Coordination: Helps brand owners maintain predictable quality and delivery cycles.
FAQ
Q1: What is your main advantage in food-grade PE injection molding?
A: Xiamen Ruicheng provides FDA-grade raw materials, full-traceability systems, controlled molding windows, and validated migration-stability performance, offering customers a practical and reliable food-contact pathway.
Q2: What information do you need for rapid quotation?
A: Food type, expected contact duration, structural drawings, quantity scale, and packaging scenario. A preliminary evaluation can be returned within 2 hours, with a complete quotation in 12 hours.
Q3: What are your MOQ and lead time for PE injection parts?
A: Trial MOQ is 50 pieces. Mass production ≥500 pieces receives tiered pricing. Standard lead time is 7–10 days, and urgent orders can be delivered within 48 hours through flexible production lines.
Q4: How do you handle quality issues after delivery?
A: A 7-day re-inspection window is provided. Confirmed quality issues will be replaced within 48 hours. Warranty coverage extends up to 18 months with documented responsibility terms.
Q5: Can you customize materials or structures for special food-contact applications?
A: Yes. Solutions can be tailored for acidic foods, high-temperature exposure, or long-term storage, including formulation adjustments and full compliance documentation.
Conclusion
Food-grade PE injection parts can meet FDA food-contact requirements when supported by certified materials, controlled processing conditions, validated cleanliness, and consistent mass-production management. Xiamen Ruicheng’s integrated solution—from material selection to compliance documentation—helps brands maintain stable, long-term packaging safety.
For expert assistance in implementing for your production needs, visit our resource center or contact us. Let’s help you scale up your manufacturing with precision and efficiency!